Research
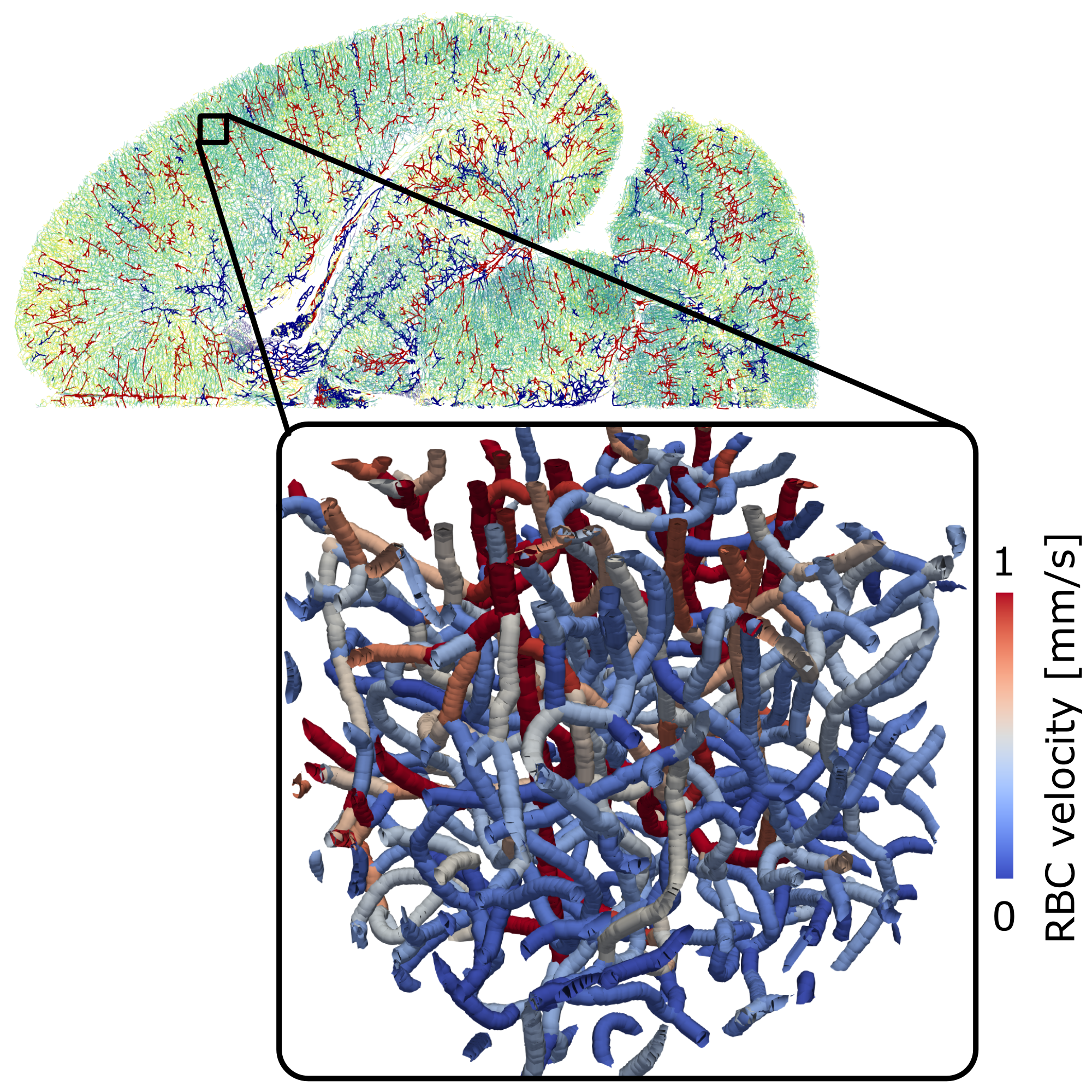
Microvascular topology and perfusion in the brain
The vasculature of the brain is responsible for ensuring a robust blood supply during baseline and elevated neural activity. While arteries are responsible for the distribution of blood to all brain areas, the capillary bed is the key location for nutrient and oxygen discharge. We utilize in silico blood flow models in realistic microvascular networks to identify key characteristics of microvascular topology and perfusion. One of our specific interests lies in the impact of perfusion heterogeneity, which is a direct consequence of the bi-phasic nature of blood (plasma and red blood cells) and the intricate microvascular structure. Another key focus lies on brain area-specific differences in topology, perfusion, and regulation. This requires an accurate labeling of arteries and veins in vascular whole brain datasets and novel in silico models to describe up-regulation of flow during increased neuronal activity.
Team member: Sofia Farina
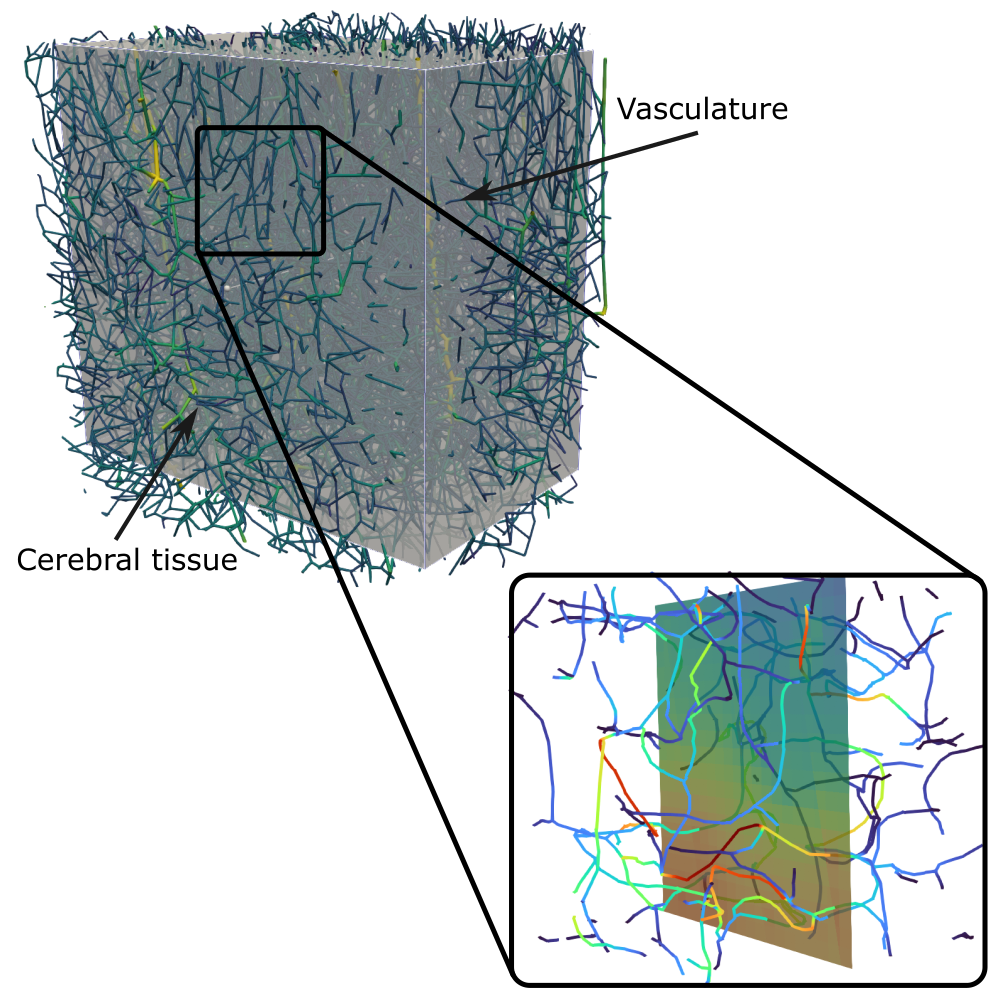
Oxygen transport
Oxygen is a key substance for the brain’s energy metabolism. It is bound to red blood cells (RBCs) and transported via the bloodstream. Perfusion heterogeneities strongly affect the oxygen discharge dynamics. Consequently, accurate numerical models need to resolve realistic microvascular structures and account for differences in discharge speed, which, for example, result from locally varying RBC volume fractions. To accommodate these aspects our model builds on mixed-dimensional modeling for the vascular and tissue domain. Our newly developed oxygen transport model will be employed to quantify the robustness of the brain’s oxygen supply during healthy conditions and pathological alterations such as reduced capillary density.
Team member: Gaia Stievano
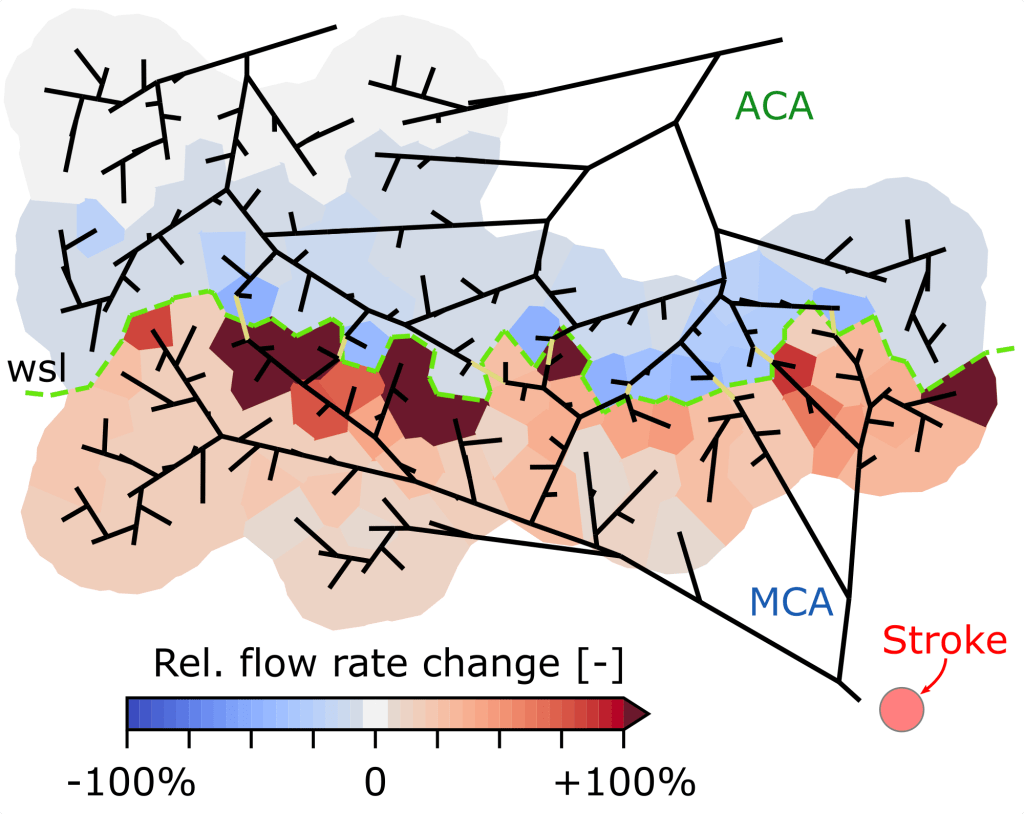
Stroke – reperfusion dynamics and treatment
Ischemic strokes, i.e., the occlusion of a major feeding vessel, affect several million people annually worldwide. Often, even after the removal of the blood clot, impairments of the patient remain. Connections between major cortical feeding vessels – so-called collaterals – have been identified to cause smaller infarct volumes. Gradual reperfusion after clot removal is another aspect associated with reduced tissue damage. To ensure a strong connection to on-going in vivo experiments, we develop numerical models able to incorporate both topological and perfusion-related in vivo data. This numerical framework is used to quantify the role of collaterals during stroke, reperfusion, and treatment and provides the opportunity to develop novel therapeutic strategies.
Collaboration with: Prof. Susanne Wegener, University Hospital Zurich
Team member: Chryso Lambride
Microstrokes
Localized microvascular alterations are a common observation during neurodegenerative disorders, such as dementia and Alzheimer’s disease. However, due to very small scales, quantifying their impact on blood flow, and oxygen and nutrient supply is challenging. The smallest microvascular alteration is the occlusion of a single capillary. After quantifying the resulting perfusion changes in a previous in silico study, we now employ inverse modeling approaches to directly align our results to in vivo experiments.
Collaboration with: Prof. Bruno Weber, University of Zurich
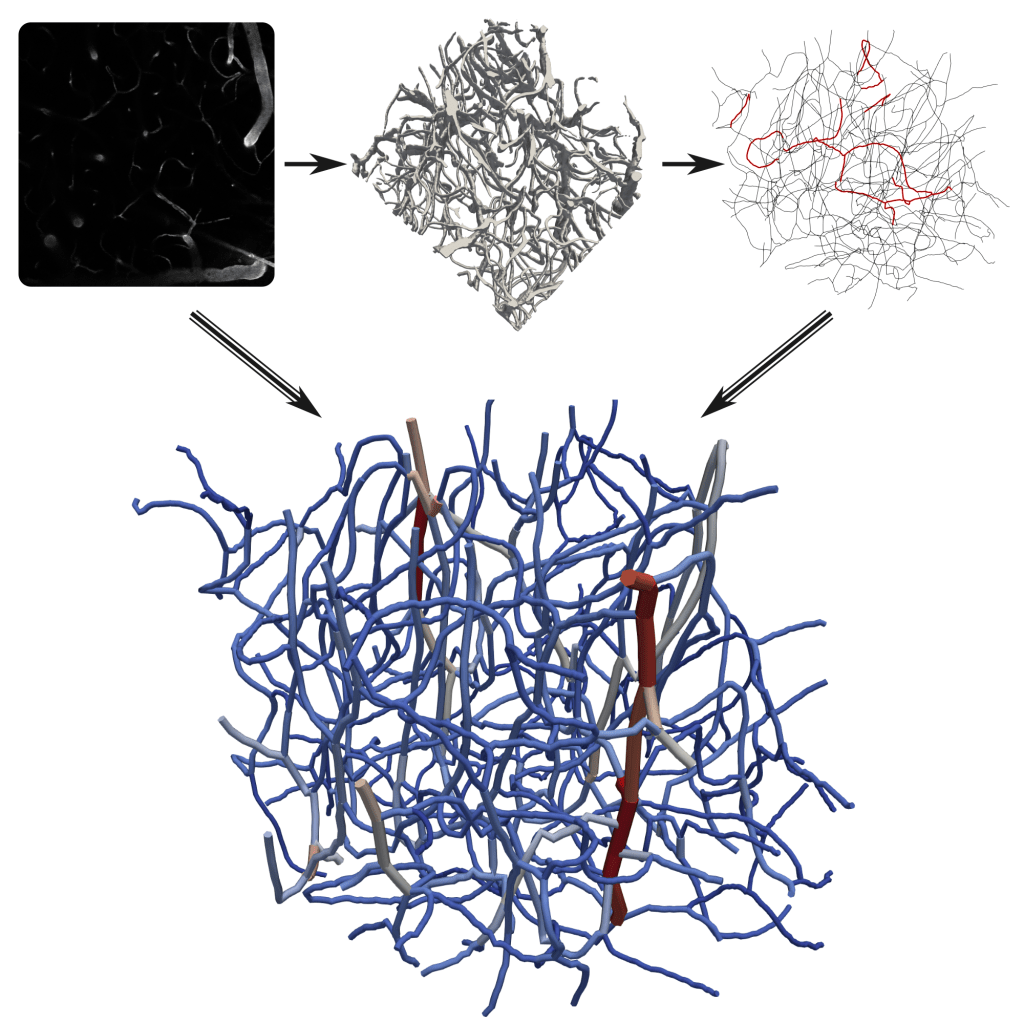

Bi-phasic blood flow modeling
A specific feature of microvascular flow is that blood particles, such as red blood cells (RBCs), are of similar size as the vessels. This causes a two-way interaction between the plasma- and the RBC-phase, which leads to both temporal and spatial heterogeneities in the flow field and RBC distribution. These interactions can be described by fully resolving RBC deformation in fluid-structure-interaction simulations or in simplified models employing empirically derived characteristics. Depending on the scale of interest, we either employ a time-averaged iterative model or a model with discrete RBC tracking. All model developments are available in our GitHub repository microBlooM.